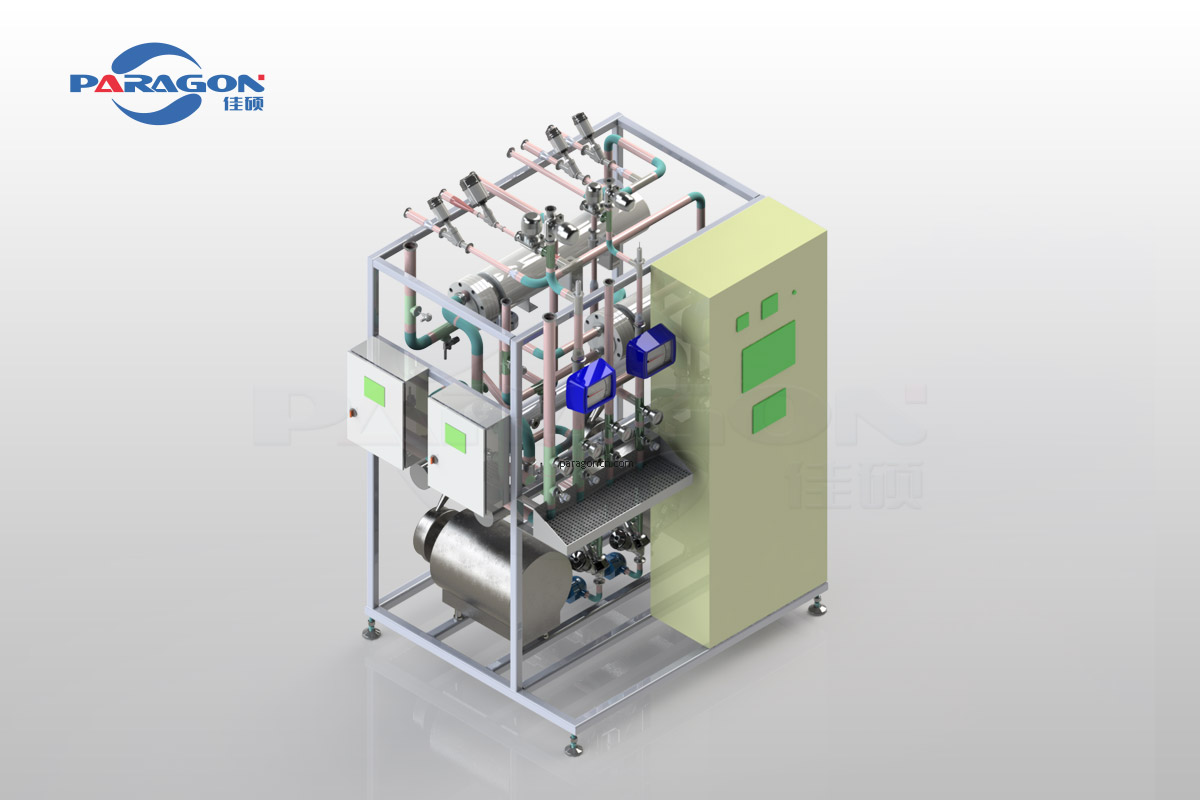
The PW SKID, designed by 3D software, has a modularized, compact, and efficient structure that is easy to operate and pleasant in appearance. We also provide customized modules depending on your needs.
You can choose from a wide range of sterilization methods, including pasteurization, ozone disinfection, pure steam sterilization, or 121°C superheated water sterilization.
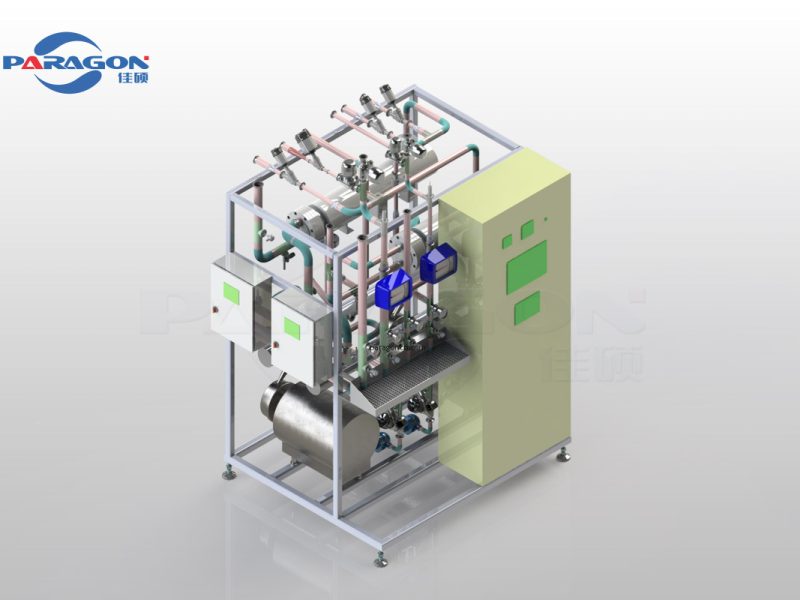
Primary components, such as pumps, heat exchangers, valves, pipes, and instruments, are sourced from world-renowned brands to ensure long-term stable operation.
In addition, the program is optimized according to GAMP 5 to achieve enhanced stability and traceability, which is supported by the documentation system.
Comprehensive assessments are conducted, including Risk Assessment (RA), Design Qualification (DQ), Installation Qualification (IQ), and Operational Qualification (OQ) in order to test the consistency of PW quality under various conditions.
The system fully complies with FDA cGMP, EU GMP, WHO GMP, and SFDA GMP.