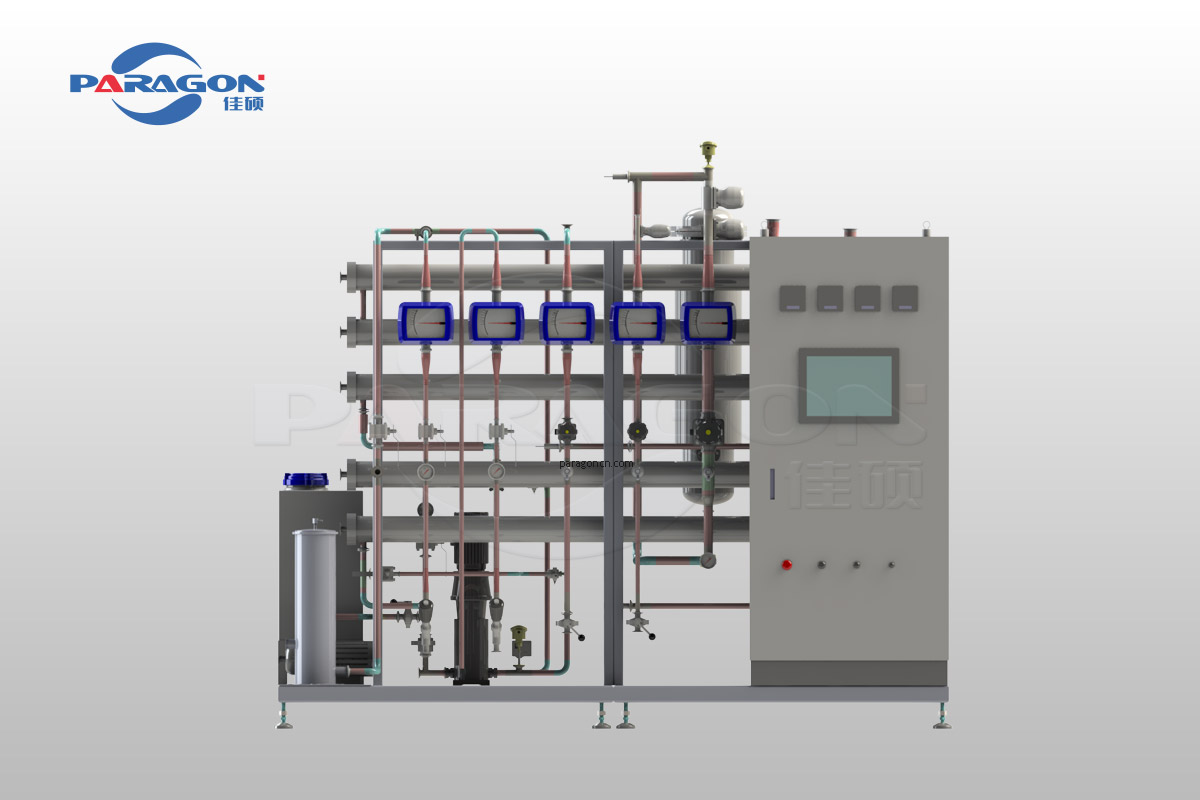
Purified water system are crucial equipment in the pharmaceutical industry, responsible for producing water that meets pharmacopoeia standards.
Water Treatment Phases in Pharmaceutical Water Systems
Operates through different water treatment phases, both related to the specific water quality and to the production necessities. The treatment phases which are needed to divide water from any organic substance, both with high or medium molecular weight, include:
- Sodium hypochloride dosing station: for water disinfection and oxidation of organic substances, reducing the bacterial charge
- Sodium metabisulphite dosing station: For neutralization and chlorine
- Double filtration system: To eliminate solid substances in inlet water
- Single or double osmotic stage: To ensure purified water through advanced reverse osmosis.
- Continous Electro-Deionizer(EDI): Ensuring the deionization of water for ultra-pure quality.
- UV lamps: For additional sterilization and deactivation of microorganisms.
Common Methods for Producing Purified Water
While WFI needs downhill ultrafiltration, purified water, traditionally produced through reversed osmosis, is commonly produced through:
- Single stage RO + Electrodeionization
- Double stage RO
- Double stage RO + Electrodeionization
Custom Water Purification Solutions for Every Need
We customize a range of purification solution packages depending on the properties of your raw water, including RO+EDI, RO+RO, and RO+RO+EDI.
Meeting Global Pharmacopeia Standards
PARAGON pharmaceutical equipment Co., Ltd. solutions produce PW (purified water) that meets the requirements of Chinese,EU, and US pharmacopeias.
Application
Purified water system is widely used in industries like pharmaceuticals, food and beverage, and cosmetics, mainly for drug formulation, equipment cleaning, and container rinsing, ensuring that every product meets quality standards.
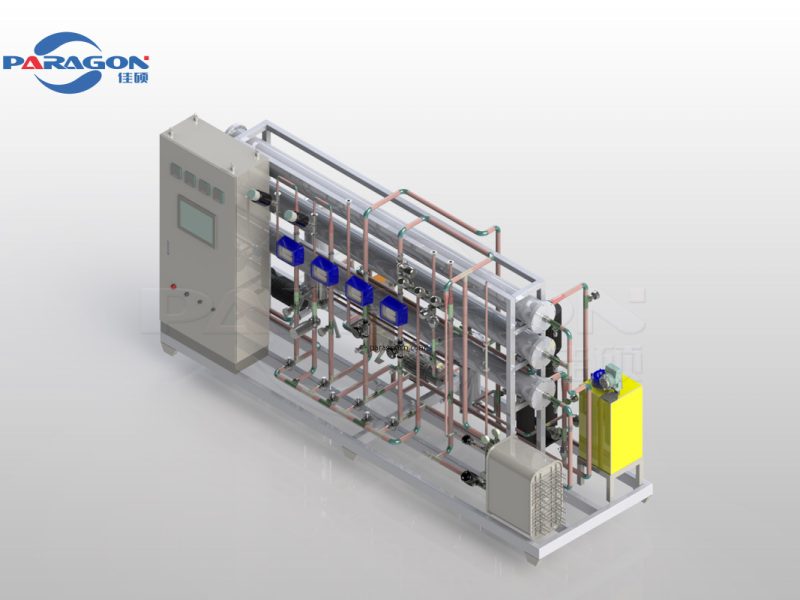
Features
- Plants are manufactured as per cGMP guidelines and complies with ASME BPE Standard
- The system features modularized production with a compact and efficient structure, making it easy to operate and maintain while requiring minimal space.
- The system has a dual-pipeline PW supply with a unique internal circulation mode, ensuring better microbial control.
- The closed-loop pasteurization mode enables thorough sterilization and precise temperature control.
- The system has a self-cleaning feature that enhances membrane performance.
- Designed for minimum dead leg criterion of < 1.5 D. Also 100% drainability can be achieved by maintaining the minimum slope of 1:100
- All contact parts are AISI 316L Stainless Steel and Non-Contact parts are AISI 304 Stainless Steel
- All contact parts surface is electro polished to less than 0.4 Ra finish.
- All interconnecting piping are orbital welded using High Purity (99.99%) argon gas and welding joints can be inspected by boroscopy machine
- Our equipment has a PLC control system and an operation system compliant with GAMP5 and 21 CFR PART 11 respectively. Various communication modes are also provided. You are free to choose from digital and paper recording devices for real-time logging of key parameters.
- Complete set of documentation and certificates to ensure compliance with regulatory authority. We provide DQ, OQ, IQ, FAT & SAT. Also assist client to develop PQ