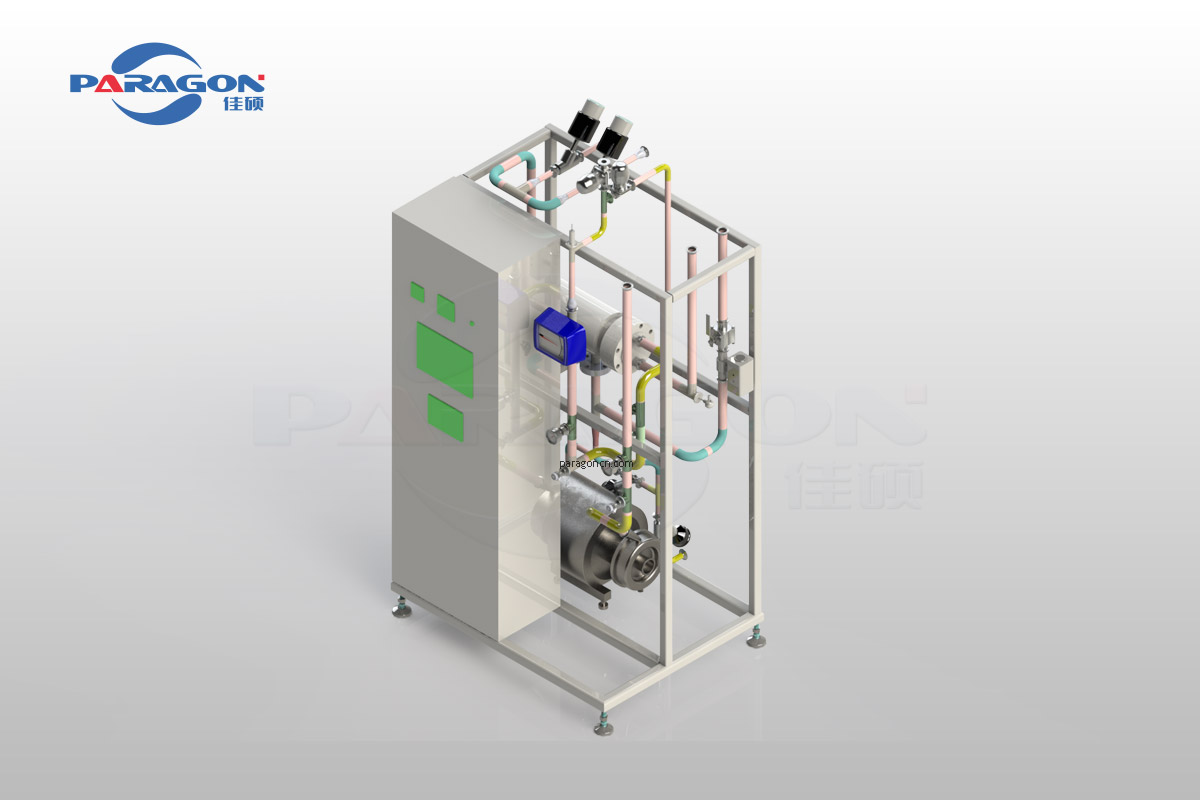
The WFI SKID, featuring a modularized and 3D design, has a compact and efficient structure that is easy to operate and pleasant in appearance.
Depending on your needs and products, the system can be configured with a variety of modes for optimal energy efficiency and lower emissions, including high-temperature storage with high-temperature
circulation, high-temperature storage with low-temperature circulation, and low-temperature storage with low-temperature circulation.

As for sterilization, you are free to choose from pure steam sterilization or 121°C superheated water sterilization. Primary components, such as pumps, heat exchangers, valves, pipes, and instruments, are sourced from world-renowned brands to ensure long-term stable operation.
The system’s program is optimized according to GAMP 5 for better stability.
Traceability is also ensured by the documentation system. To guarantee the system can reliably produce high-quality WFI under various conditions, assessments are conducted, including Risk Assessment (RA), Design Qualification (DQ), Installation Qualification (IQ), and Operational Qualification (OQ). The system is fully compliant with FDA cGMP, EU GMP, WHO GMP, and SFD A GMP.